The Discngine Chemistry Component Collection is an extension to the standard Chemistry package for Pipeline Pilot.
Pharmacophore Graph
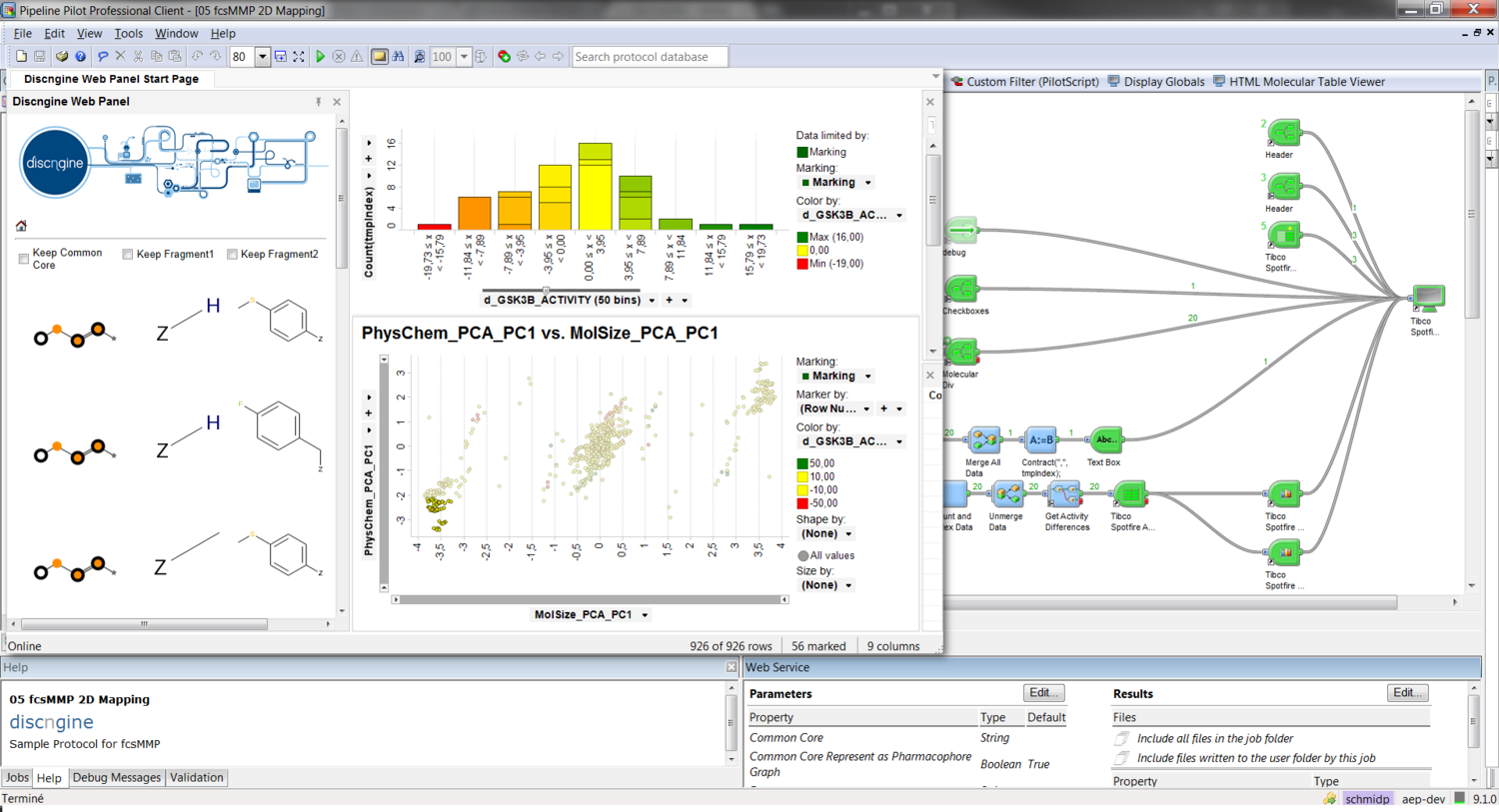
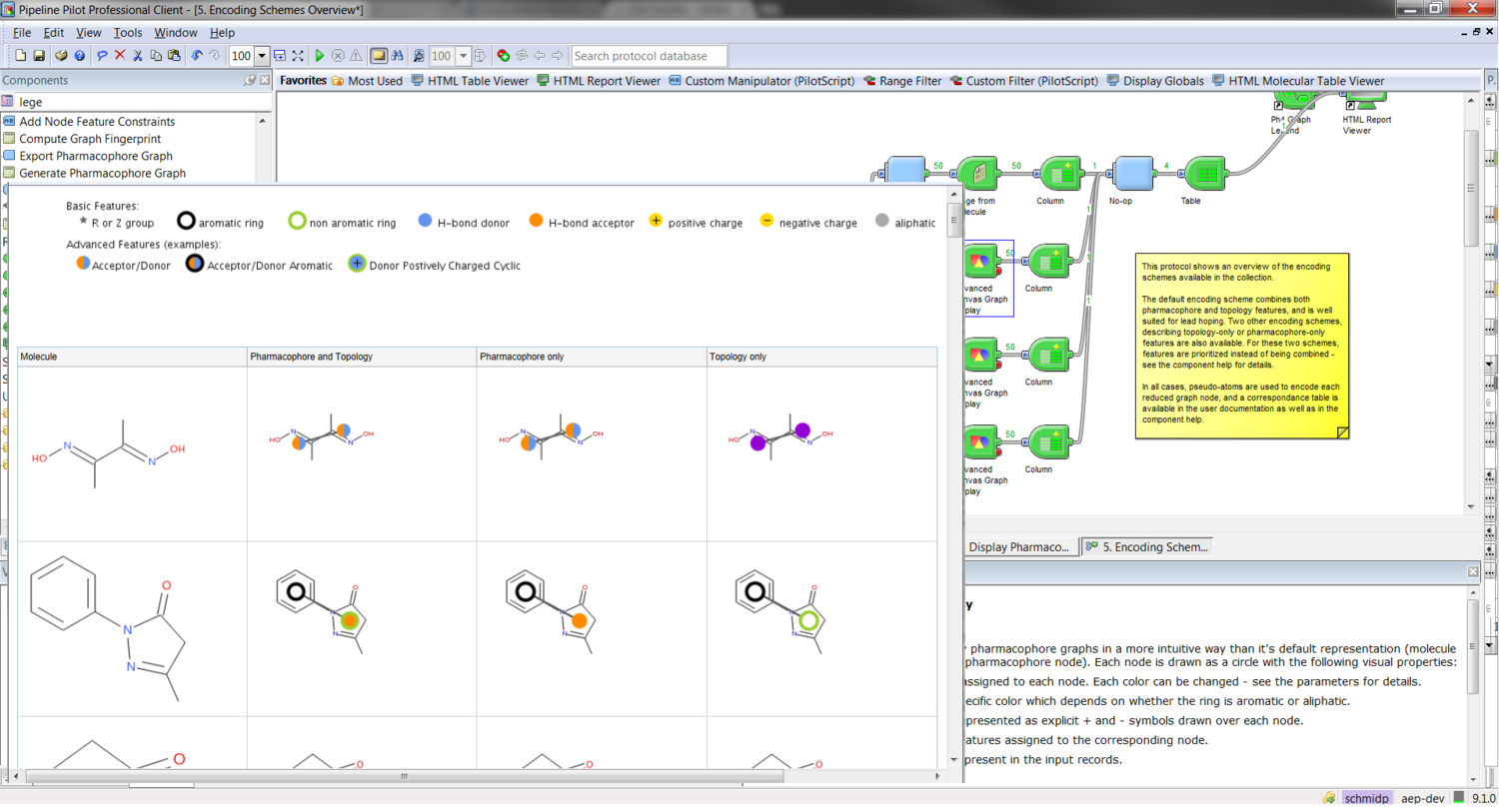
Enable reduced graphs within Pipeline Pilot using the Discngine Chemistry Collection. Build pharmacophore graphs using various encoding and reduction schemes, compare them, query a collection of graphs or align similar molecules in 2D and 3D. Derive leaphopping and lead optimization applications for your medicinal chemists with a minimum amount of work.
Ease your cheminformatics work
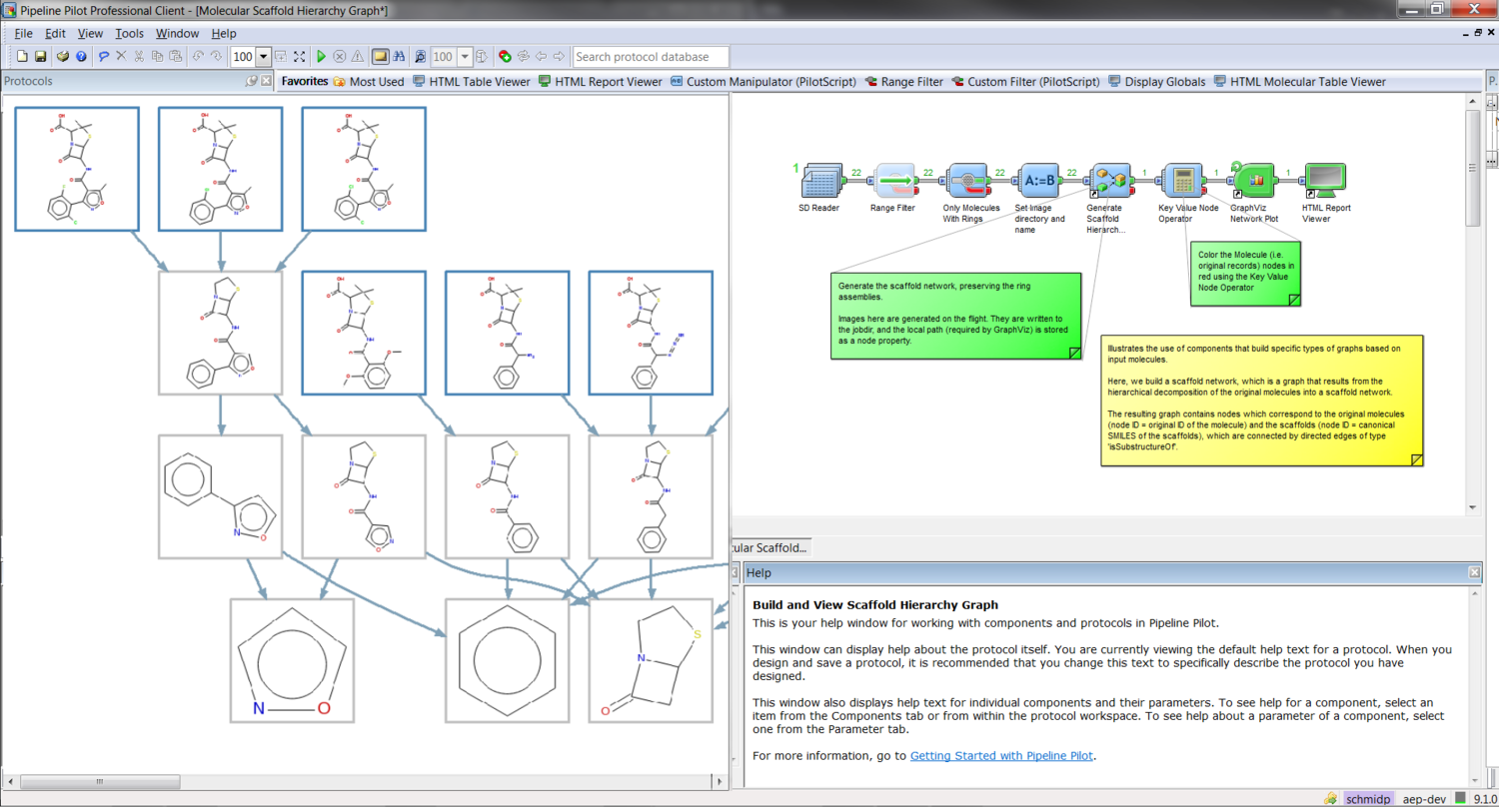
The collection is shipped with components and protocols allowing advanced fragmentation and descriptor calculation. Create interactive scaffold networks with a few clicks and use our fuzzy pharmacophoric triplets to screen your compound databases. Start playing with the sample applications and get productive right from the beginning.
Fuzzy Matched Molecular Pairs
Readybuilt Pipeline Pilot components allow you to de- rive statistically validated & more precise transformation rules compared to classical MMP. For the first time, perform MMP analysis on small to medium sized datasets on target activity data. Build powerful web applications for your MedChem community using the sample protocols of the Discngine Chemistry Collection for Pipeline Pilot.
In the Literature
Hit expansion approaches using multiple similarity methods and virtualized query structures. - JCIM
“The Chemistry Collection is wicked cool. It has a ton of stuff. I can see all the hard work in there.”
Key Features
Fuzzy MMP Analysis using Pharmacophore Graphs to enable MMPA for smaller datasets and improved statistical significance
Highly customisable reduced graph representation to perform lead hopping
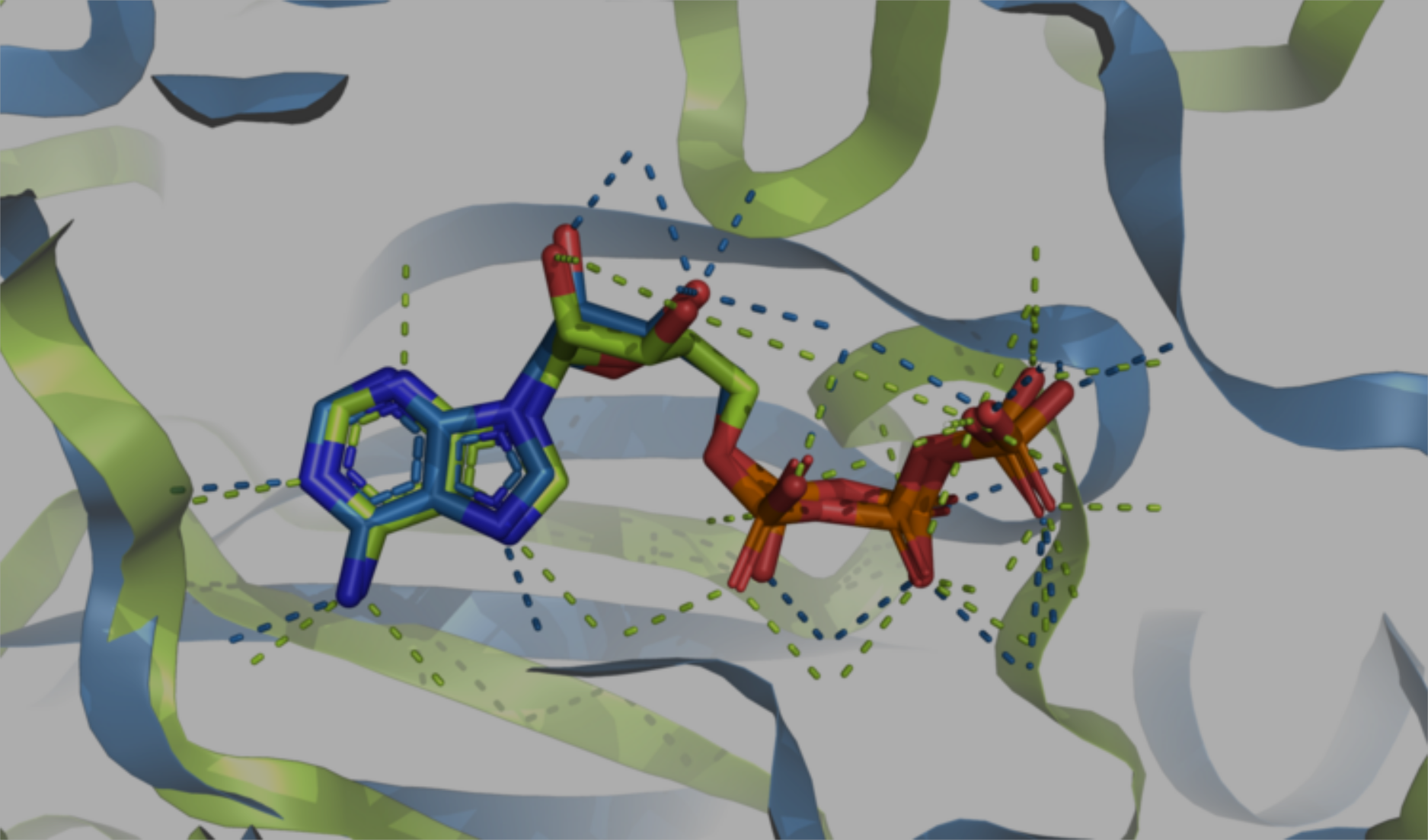
3D Pharmacophore Graph components allowing matching & filtering
Out-of-the-box scaffold networks and recursive molecular fragmentation
Scientifically validated Fuzzy Pharmacophore Triplet Fingerprints